A review of the health effects of isocyanates in casting work

Summary
Isocyanates are a leading cause of occupational asthma worldwide. Isocyanates are known to be powerful irritants and sensitizers of the skin and the respiratory tract. The health effects can manifest as occupational asthma, difficulty in breathing and chest tightness, direct skin inflammation and persistent or recurring irritation of the eyes. The increased production of isocyanate containing consumer products can lead to increased nonoccupational exposure while e.g. using sprayed polyurethane foams for insulation at home.
The hazardous health effects of isocyanates appears to be well established and globally well-recognized. Many authorities have set occupational exposure limits to diisocyanates. In Europe, many authorities have advised to avoid the use of isocyanate and to use comparable chemicals whenever possible. While preventive exposure limits and use of less volatile forms of diisocyanates have reduced the potential for respiratory exposure, occupational asthma still occurs. Occupational asthma is observed also in work settings where isocyanate respiratory exposures are low or not detected, but where is potential for dermal exposure.
There is a lack of recognition of isocyanate exposure. The risk assessment in industrial sites handling isocyanates has been shown to be worse compared to industrial sites handling other chemicals. In a study by the Finnish TTL, isocyanates were found to be the causing agent in almost all significant risk cases in rubber and plastic production.
There is a certain complexity in managing exposure to isocyanates. Isocyanate exposure cannot be detected by human senses. The downstream users neither sales representatives are not aware of the potential health hazards of isocyanate exposure and therefore the relationship between exposure and symptoms can remain unconnected. The risk of isocyanate exposure is often well-known when working with high exposure products, e.g. spray painting. However, the potential risks are not recognised in products containing minor concentrations of isocyanates. Similarly short-term and occasional exposure can be thought to be less significant. There are detection limits in exposure measurements, and therefore isocyanate exposure can be non-detectable although it exists. Furthermore, sensitised subjects can change assignments or employers without reporting occupational exposure to authorities. In fact, the Finnish TTL has estimated that the actual number of occupational asthmas could be 2-3 times higher.
Risk of occupational exposure to isocyanates appears to be better recognised when working with high respiratory exposure products, e.g. spray painting. In such working environments, protecting equipment are usually used. Regardless, the skin exposure is often not recognised or is not considered substantial. In studies investigating protection again dermal isocyanate exposure, more than half of the workers did not use gloves or used gloves that were not intended for chemical protection.
Several cases of isocyanate related occupational asthma concerning workers handling orthopaedic plaster casts have been reported. Today, the majority of orthopaedic casting work is made using plaster casts, which all contain diisocyanate MDI. In a few measurements done, the airborne exposure to MDI has found to be low during casting work. Nevertheless, the low airborne concentrations of MDI detected during casting work and several occupational asthmas observed would suggest that dermal exposure could be the main source of MDI in casting work. In fact, unawareness of the potential risks of casting products among casting workers results in inappropriate protection against dermal exposure to MDI. It has been shown that the most common working clothing and gloves easily permeate MDI. Due to lack of knowledge, MDI permeable gloves and work cloth can be used during casting.
1. Chemical properties and identity of isocyanates
Isocyanates are highly reactive chemicals which contain the functional isocyanate group R–N=C=O. Isocyanates are classified based on the number of reactive groups and on their molecular structure. All isocyanates contain at least one isocyanate group; monocyanates contain one, diisocyanates contain two groups and polyisocyanates multiple. Isocyanates can be divided into aliphatic, cycloaliphatic and aromatic compounds, according to their molecular structure. Examples of molecular structures of isocyanates are shown in Figure 1.
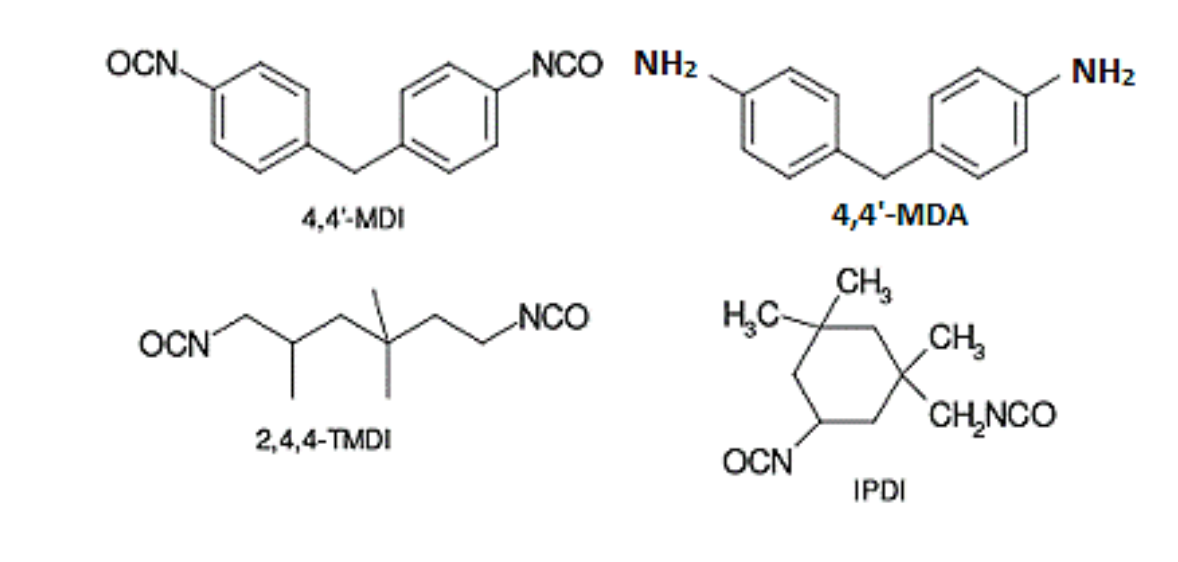
The reactivity of the NCO group varies in different isocyanates depending on the other molecular structures attached to it. Another functional group attached to the NCO group may change its three-dimentional structure and e.g. lipophilic properties. Due to varying reactivity, isocyanates have different properties in biological systems. Such properties determine molecules’ permeability through biological membranes and their ability to reach the target reaction sites. The moiety attached to the NCO group appears to be responsible in part for variations in biochemical reactivity and toxicity within the isocyanate class. (Bello et al. 2004)
As diisocyanates have reactive groups in two positions, it allows them to act as a cross-linker in polymerization reactions to form polyurethanes or polyisocyanates. Monoisocyanates are less reactive and are less commonly used for industrial purposes. (Wisnewski et al. 2010)
1.1 Diisocyanates
Diisocyanates are a group of chemicals out of which more than half are liquids and the rest are in powder form at room temperature. All of them are combustible and soluble in most of organic solvents. Diisocyanates react highly with OH groups e.g. with alcohols and water, including moisture in the mucous and membranes, to form inert, solid and insoluble polyurea (Hoffmann et al. 2010, European Commission 2010. The most commonly used diisocyanates include diphenylmethane diisocyanate (MDI), toluene diisocyanate (TDI), hexamethylene diisocyanate (HDI) and isophorone diisocyanate (IPDI). (Bello et al. 2004, CDC 2014)
Diisocyanates are the raw material for forming all polyurethane polymers, which are compounds of e.g. flexible and rigid foam, spandex fibers and polyurethane paints. Isocyanates are thereby used for example in insulation material, car seats, furniture, foam mattresses, synthetic leather, packing material, polyurethane rubber and spray-on polyurethane protecting wood and fiberglass. In healthcare, isocyanates are found e.g. in all synthetic casting tapes, endoscopes and catheters. (TTL Jan 2013, Abbott 2013, CDC 2014) Production of isocyanates has rapidly increased in the past and is predicted to increase at an annual rate of around 5%. Consumer products and construction are the main drivers of growth. (Vershoor et al. 2014)
1.1.1 Diphenylmethane diisocyanate MDI
MDI has become the most commonly produced and technically used isocyanate in the world (Henriks- Eckerman et al. 2015, Wisnewski et al. 2015). It is used in all orthopaedic plaster (fiberglass) casts (Abbott 2013). Thereby this report further focuses on MDI and its biological metabolites.
MDI or pure MDI is a mixture of primarily 4,4’-MDI with small amounts of 2,4’ ja 2,2’ isomers. The primary commercial form of MDI is polymeric MDI (PMDI), which is a mixture containing 25-80 % of monomeric MDI and oligomeric polyisocyanate homologues of MDI. Oligomeric polyisocyanates consist of at least three NCO- groups. (WHO 2000, Henriks-Eckerman et al. 2012) Monomeric MDI is solid at room temperature, whereas PMDI is liquid at room temperature (NIOSH 2010).
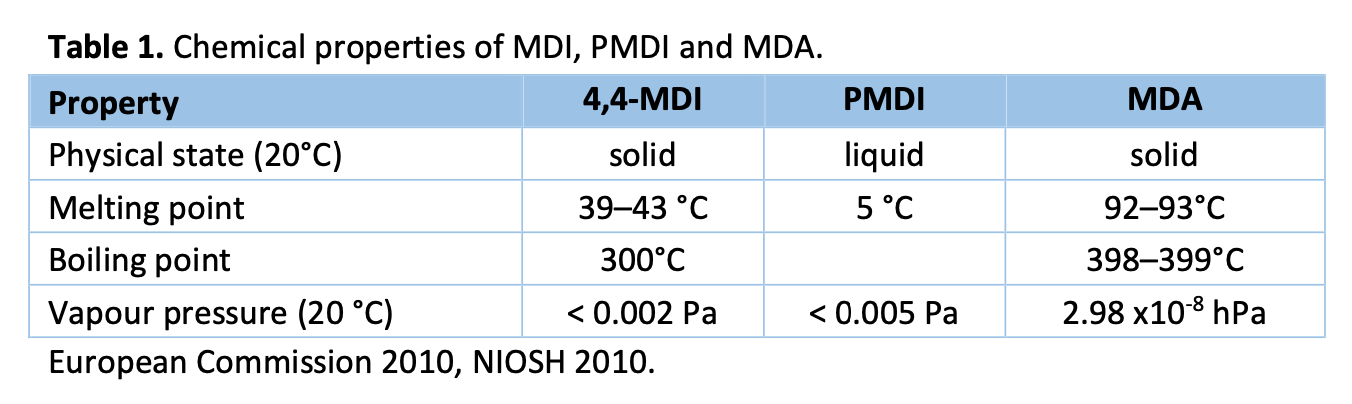
Compared to other isocyanates, MDI is relatively lipophilic and therefore likely to penetrate biological membranes easier and reach biologically susceptible sites faster (Bello et al. 2004). In organisms, MDI is highly reactive with water and is rapidly hydrolysed (half-life of ~20s) to form 4,4'- methylenedianiline (MDA)(see figure 1). MDA reacts with excess MDI to form insoluble oligoureas and polyureas, or is converted to adducts with proteins and other biomolecules (Hoffman et al. 2010). Studies of workers have identified free MDA, acetylated MDA and adducts to either haemoglobin or albumin in urine and blood. (WHO 2000) The biological half-life of MDA in humans ranges from 50 to 80 hours. (WHO 2000, Pearson et al. 2013)
Global production of MDI and polymeric MDI is over 5 million tonnes per year (Mt/a in 2011). More than 50 % of the production of isocyanates consists of MDI. (Wikipedia) In Europe, MDI is produced and further processed to polymers in 11 industrial sites (European Commission 2010). In Finland, there is no production of MDI, but it is the most commonly used isocyanate. The import of aromatic diisocyanates exceeds 1,200 tonnes annually and most of the imported diisocyanate is PMDI (Henriks-Eckerman et al. 2012).
2. Human exposure to isocyanates
2.1 Health effects
Isocyanates are known to be powerful irritants to the mucous membranes, the skin, the eyes and the respiratory tract (NCDOL 2013, CDC 2014, Hoffmann et al. 2010). The hazardous exposure can manifest as occupational asthma, difficulty in breathing and chest tightness, direct skin inflammation and persistent or recurring irritation of the eyes. Also death from severe asthma in sensitised subjects has been reported (NCDOL 2013, CDC 2014).
The health effects of diisocyanates include (Bello et al. 2004, HESIS 2014, WHO 2010, TTL Jul 2013, European Commission 2010)
- Contact dermatitis
- Skin and respiratory tract irritation
- Immune sensitisation
- Asthma and other pulmonary diseases
- Carcinogenicity
- Mutagenicity
- Toxicity for reproduction
Isocyanates have different toxicological properties due to the differing reactivity of the NCO functional group. Due to the varying reactivity, the toxicological potential of isocyanates also varies. (Bello et al. 2004) Exposure hazards of isocyanates are directly related to volatility (tendency to vaporise) and molecular weight. Isocyanates with low volatility and larger molecular weight, such as MDI, are less toxic than isocyanates with low molecular weight, such as TDI. (Sullivan and Krieger 2001)
2.1.1 Sensitisation and irritation
Isocyanates are powerful irritants and sensitizers of the skin and the respiratory tract. High airborne concentration of isocyanates can cause acute irritation of the respiratory tract and the eyes, occurring as difficulty in breathing and chest tightness, burning, itching, rhinitis and cough. The severity of irritation symptoms correlates with the level of exposure. (Bello et al. 2004, European Commission 2010, HESIS 2014)
The signs of respiratory tract and skin irritation can occur during the exposure, soon after the exposure or with delay. Some workers who are exposed to isocyanates can become sensitized. Both respiratory and dermal exposures can lead to sensitization (CDC, 2014). Sensitization to isocyanates can occur immediately after exposure or as a late allergic reaction. In sensitized workers, even small exposures can cause skin or lung reactions.
Allergic contact dermatitis and delayed contact dermatitis are usually T-lymphocyte mediated reactions, which occur after repeated exposure. In Finland, approximately 20 diisocyanate related occupational allergic contact dermatitis or delayed contact dermatitis were diagnosed between 1998 and 2010. Most of the cases were diagnosed in subjects working in the manufacturing of vehicles, paints or electronic devices or in construction or painting. (TTL Jul 2013, TTL Chemicals at work 2005)
2.1.2 Acute toxicity
Isocyanates have acute toxicity in high exposures. The main target organs are the eyes and the respiratory tract. Acute toxicity manifests as irritation and sensitisation symptoms: irritation of the eyes, nose and throat, the respiratory tract, chest pain, dyspnea and asthma. (NIOSH Pocket Guide to Chemical Hazards, 2010) There is limited data from animal studies and data from the Bhopal chemical disaster. The Bhopal disaster in India in 1984 is one of the worst chemical disasters in history; more than 3,800 people died within one hour and an estimated 10,000 in the first following days after a massive methyl isocyanate gas leakage of 40 tons. The Indian government reported that more than 500,000 people were exposed to the gas. According to retrospective evaluation, subjects exposed to high concentration of isocyanates suffered from severe and persistent irritation of the skin and the eyes. (Broughton 2005) Chronic inflammatory damage of the eyes and the respiratory tract appeared to be the main cause of morbidity (Dhara 1992).
2.1.3 Occupational asthma
Isocyanates have been the leading chemical cause of occupational asthma worldwide for several decades. (Mhike et al. 2013, Henriks-Eckerman et al. 2015, Wisnewski et al. 2010) Most of the asthma cases are related to MDI or HDI exposure (TTL Jul 2013).
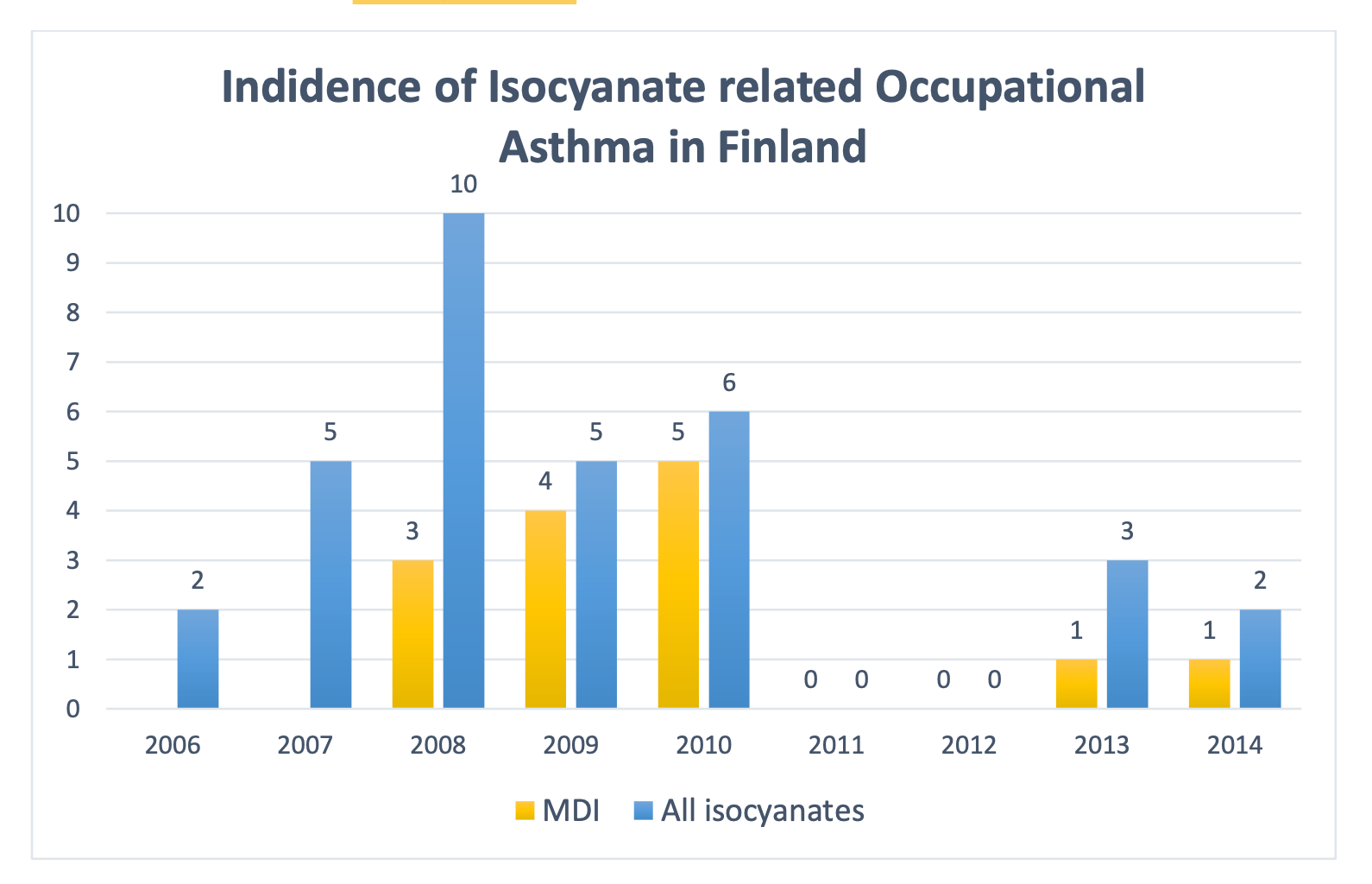
Chemicals cause approximately 400 respiratory allergies in Finland annually, including asthma, allergic rhinitis and allergic alveolitis. Out of these, less than 10 cases are reported as isocyanate related after evaluation, and 2-3 cases are reported to be related to MDI (see figure 2). The incidence of isocyanate related asthma in Finland between 2006 and 2014 is shown in figure 2. The incidence of occupational asthma related to isocyanates has decreased significantly since the 1980’s, when 20-30 new cases were diagnosed annually in Finland. In Germany, 80-120 new allergy and asthma cases related to isocyanates are diagnosed annually (taustaa, Germany). Most of the occupational asthmas are diagnosed in shipwrights, car mechanics, insulation workers and casting workers. (TLL Jul 2013)
Figure 2. The incidence of isocyanate related occupational asthma in Finland between 2006 and 2014. (Finnish TTL, Irmeli Lindström)
Possible mechanisms behind isocyanate related asthma
Isocyanate related asthma was first recognized over 60 years ago, yet the mechanisms behind it remain unclear (Wisnewski et al. 2015). The health effects of isocyanates can be mediated by humoral and cellular mechanisms. Both immediate and late effects are known to appear. The specific humoral immune response can be mediated by IgE and IgG, but interestingly not all sensitized patients have demonstrative serum antibodies. (WHO 2000) This has also led to the fact that the diagnostic testing for isocyanate specific asthma is not sensitive (Mhike et al. 2013).
Binding of the reactive NCO group to several human proteins in airway epithelial cells, serum and skin is suggested to be an important step leading to isocyanate allergy, asthma and sensitization (Bello et al. 2004, Wisnewski et al. 2015, Mhike et al. 2013). Isocyanate-albumin adducts have been found circulating in peripheral blood of exposed workers. (WHO 2000, Wisnewski et al. 2015) Also binding to other proteins, e.g. keratin, tubulin, glutathione and haemoglobin, has been suggested. (Mhike et al. 2013). Antibodies triggered by isocyanate exposure specifically recognize isocyanate conjugated albumin but no other carrier proteins. This suggests that albumin may be the major reaction target for isocyanate in vivo. However, isocyanate- albumin specific IgE antibodies are commonly undetectable among hypersensitive individuals. (Wisnewski et al. 2015).
2.1.4 Carcinogenicity, mutagenicity and toxicity to reproduction
The European Chemicals Agency (ECHA) has classified MDI as a possible carcinogenic substance (see table 2). A few studies have suggested isocyanates causing several types of cancer, such as prostate cancer, but those have not been confirmed (WHO 2000).
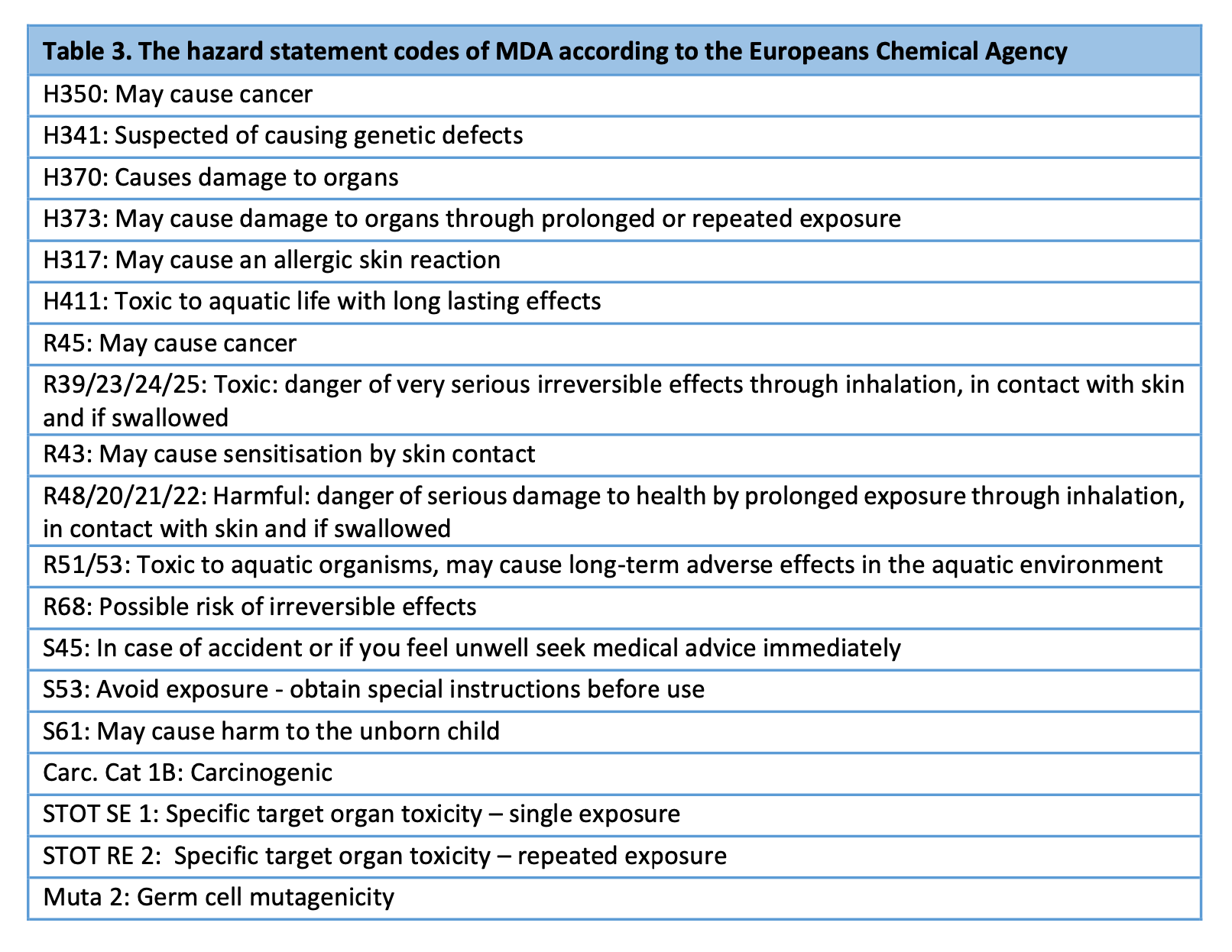
Based on the effects observed after isocyanate disasters, isocyanates have been suggested to affect reproductive health (Broughton 2005, Dhara 1992). This might be related to the biological metabolites of isocyanates. Also animal data has suggested toxicity for reproduction (WHO). Mutagenicity or toxicity to reproduction have not been confirmed to MDI as there is no data on reproduction toxicity available. However, the ECHA has classified the biological metabolite of MDI, methyldiamine MDA, as mutagenic and toxic to reproduction (see table 3).
2.1.5 Hazard statement codes of MDI and MDA
The ECHA has classified MDI, a diisocyanate used in all synthetic plaster casts, as harmful, irritant, sensitising and carcinogenic. The hazard statement codes are presented in table 2).
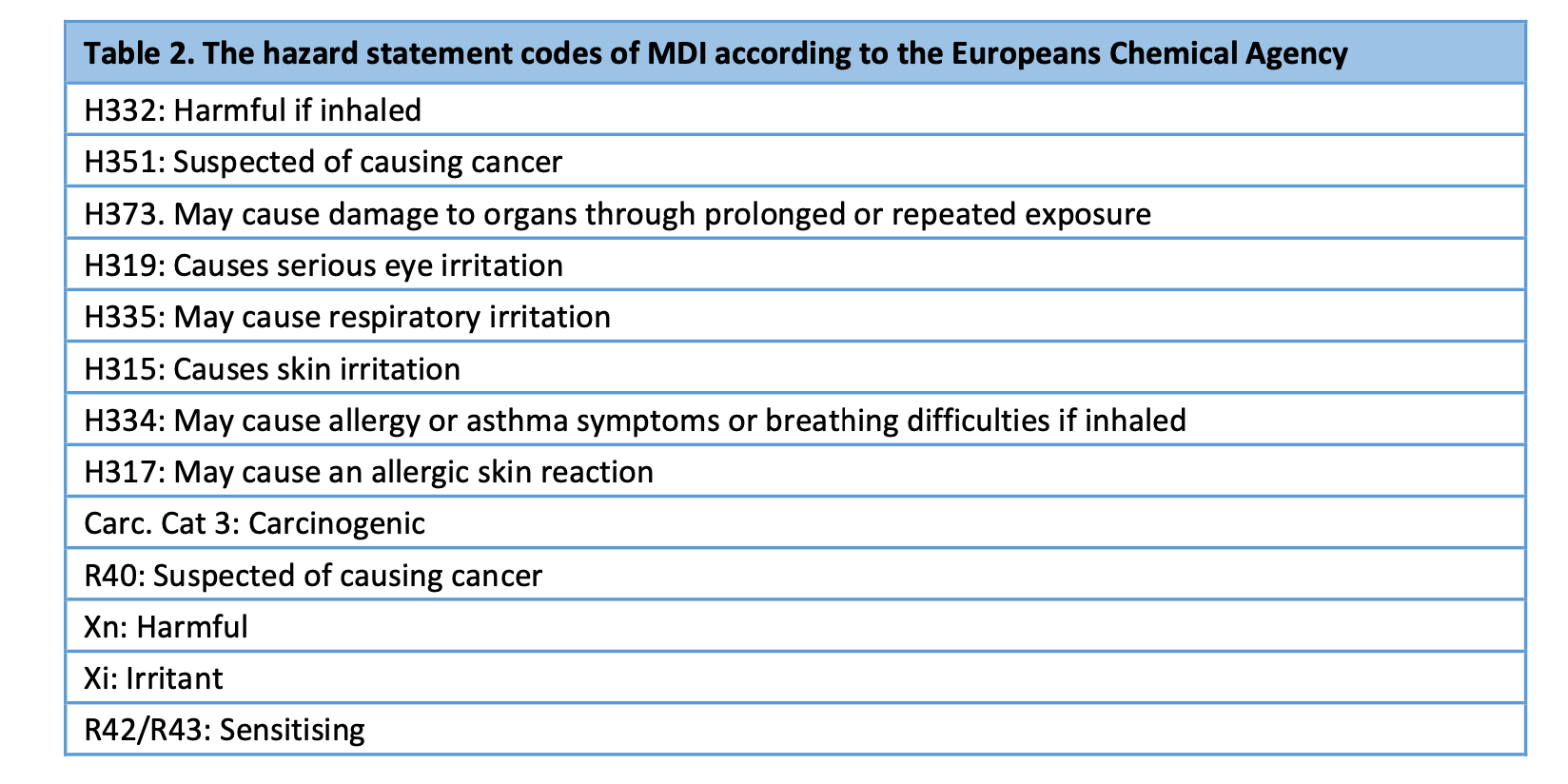
MDA, a biological metabolite of MDI, has an even longer list of hazard statement codes than MDI according to the ECHA: MDA is classified as i.a. carcinogenic, mutagenic and as causing target organ toxicity (see table 3). Importantly, MDA is classified as possibly causing harm to the unborn child and as causing germ cell mutagenicity.
2.2 Exposure routes
Exposure to diisocyanates may occur by inhalation of vapours and aerosols, ingestion and skin or eye contact. The respiratory and dermal toxicity of isocyanates is well established both in humans and in laboratory animals. (WHO 2000, TTL Chemicals at work 2005)
The respiratory system is the main target for isocyanate toxicity (Sullivan and Krieger 2001). Inhalation has long been considered the primary route of isocyanate exposure. Research and regulation are focused on measuring and preventing respiratory exposures. Globally, use of less volatile forms of diisocyanates, such as MDI, is preferred (Liljelind et al 2010). Due to low volatility and vapour pressure, the airborne concentration of MDI is expected to be low (WHO 2000). Volatility and vapour pressure of a chemical are directly related to inhalation potential. The potential for respiratory inhalation of MDI increases as temperature rises. The reaction of the isocyanate group and the OH group is exothermic, which amplifies evaporation of MDI. Nevertheless, the proportion of vaporised MDI is expected to be low due to low vapour pressure (European Commission 2010).
While improved control and use of less volatile forms of diisocyanates have reduced the potential for respiratory exposure, occupational asthma continues to occur (Liljelind et al. 2010). Occupational asthma is observed also in work settings where isocyanate respiratory exposures are low or not detected, but where is potential for dermal exposure (Bello et al. 2007). Therefore skin contact may be the major route of exposure of diisocyanates (Bello et al. 2004). Interestingly, there is evidence that dermal exposure can induce asthma and other respiratory allergy symptoms (Bello et al. 2004, Suojalehto et al. 2011, Henriks-Eckerman et al. 2015).
2.2.1 Occupational exposure levels
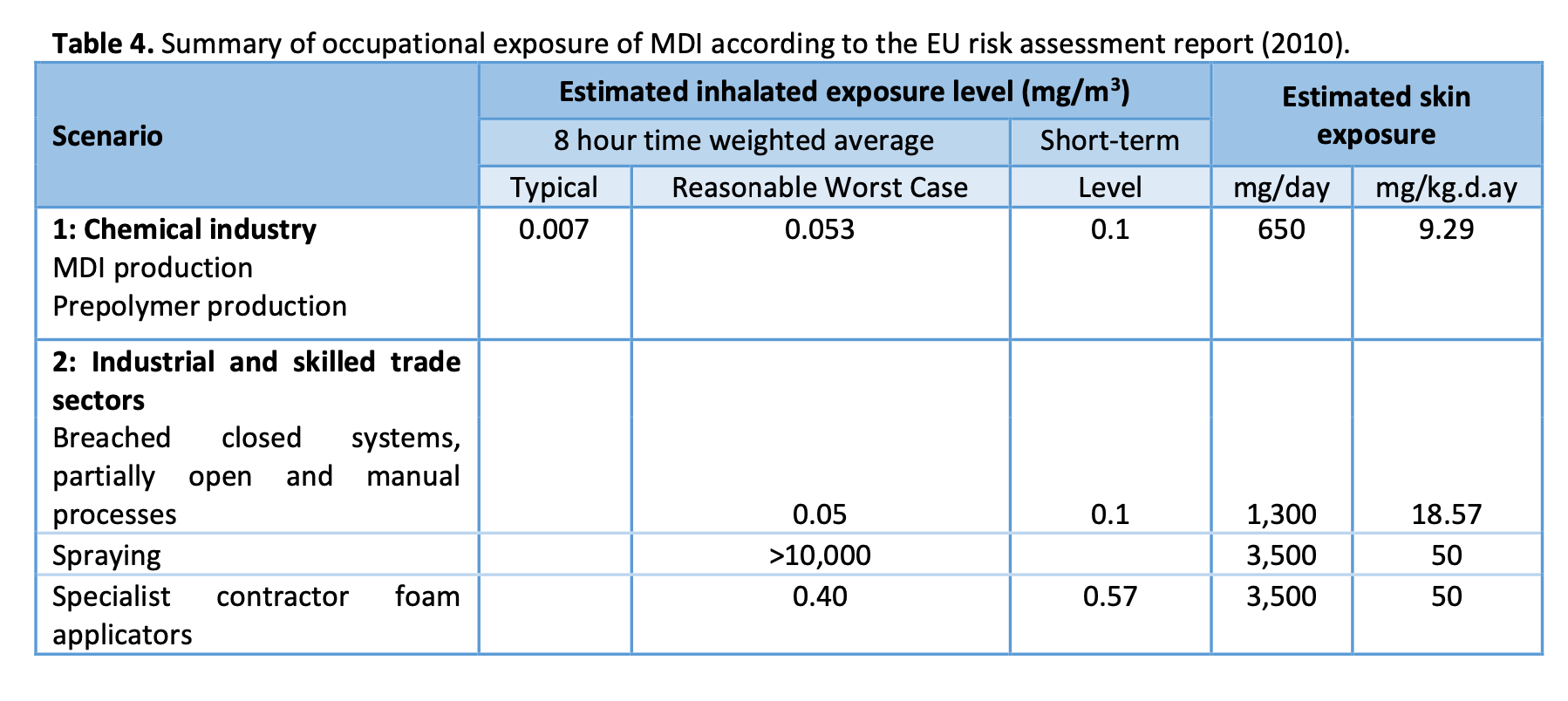
Some estimates about occupational exposure during production of MDI and use of MDI have been presented in the EU risk assessment report of MDI. In table 4, exposures are shown in two scenarios: scenario 1 represents production in closed systems and scenario 2 intermediate use of MDI by the downstream users. As shown in the table, potential exposure levels are not well established in different scenarios.
In a real-life setting, Creely et al. (2006) measured occupational airborne concentrations of isocyanates in a variety of workplaces in the non-motor vehicle repair sector. Only companies judged to be using good working practices where selected to participate. Air concentrations were measured from 22 sites in 213 companies by personal monitoring. The airborne concentrations were found to range between 0.5-66 μg/m. The highest inhalation exposures were measured during spray painting and spray application of polyurethane foam insulation, in both above the UK workplace exposure limit.
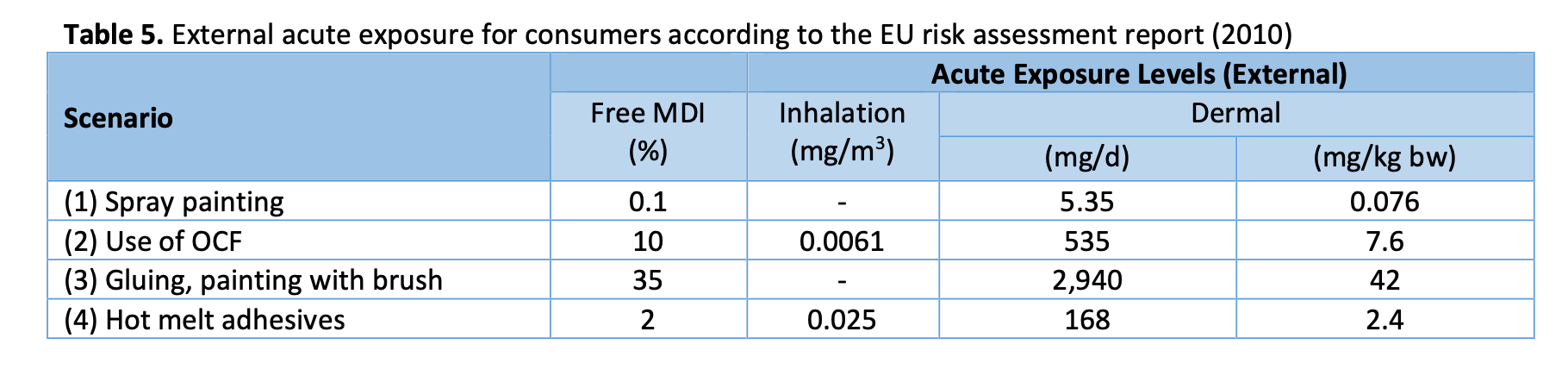
As a reasonable worst-case consideration, a person working with isocyanate products also at home can receive remarkable daily dose of MDI. Table 5 shows the main results of the consumer exposure assessment shown in the EU risk assessment report of MDI (2010). Scenario 1 represents spray painting, e.g. liquid roof coating. Scenario 2 represents use of spray foam or one component foam. Scenario 3 shows gluing and the use of putty/filler in cartridge and painting with brush. Scenario 4 represents use of hot melt adhesives.
Scenario 3 can be considered the situation most similar to orthopaedic plaster casting, as brush painting and gluing involve hand contact similarly as casting. Still, the proportion of free MDI in casting materials (approx. 8 %) is expected to be less than in glue and paint (Material Safety Data Sheet 3M, 2009).
Inhalation has long been considered to be the primary route for diisocyanate exposure. However, there is growing evidence that skin exposure can be an important route of isocyanate sensitisation (Bello et al. 2004).
In animal studies, the most commonly used diisocyanates, HDI and MDI, have been observed to induce airway hypersensitivity through the dermal exposure route (Liljelind et al. 2010).
Only a few studies on dermal exposure to isocyanates have been made, as studies often focus on measuring airborne concentrations. The Finnish Institute of Health has measured workers’ dermal exposure to chemicals at different industrial sites (Henriks-Eckerman et al. 2012). By using the tape-strip method, the mean dermal concentration of MDI was 0.1-2 μg/ 10cm . In another study by Henriks-Eckerman et al. (2015), the tape-strip measurement results were below 2 μg/10cm2 from the skin of the hands and arms among almost all workers handling MDI manually in construction work and the boat building industry, regardless of mixing methods, glue or foam application methods or whether gloves were used.
Liljelind et al. (2010) measured dermal exposure to MDI in iron foundry using the tape-strip technique. The core makers had an average exposure of 0.77 μg/10cm2 measured from their hands, arms, wrists and foreheads. Henriks-Eckerman et al. (2015) note that the face may contribute to the majority of the total dermal exposure to MDI due to its large, usually uncovered area. So far only Liljelind et al. (2010) have measured samples from workers’ foreheads, where the highest measured MDI concentration was 0.972 μg/10cm.
Even though the tape-strip method can be used to measure MDI exposure even when airborne concentrations are unquantified, the technique may not be able to demonstrate the absolute dermal exposure. It has been found that some MDI may be absorbed into or adhere to the skin so that it cannot be stripped off (Henriks-Eckerman et al. 2015). In animal studies, considerable amounts of applied labelled MDI were found at the application site which could not be washed off (Hoffmann et al. 2010). As a consequence, dermal exposure to MDI may be greater than what can be measured with the tape-strip method. In fact, the skin exposure to MDI in production or intermediate use of MDI are estimated to be significantly higher in the European Risk assessment report of MDI than those levels detected by the tape-strip method (see table 4)(European Commission 2010).
2.2.2 Environmental exposure
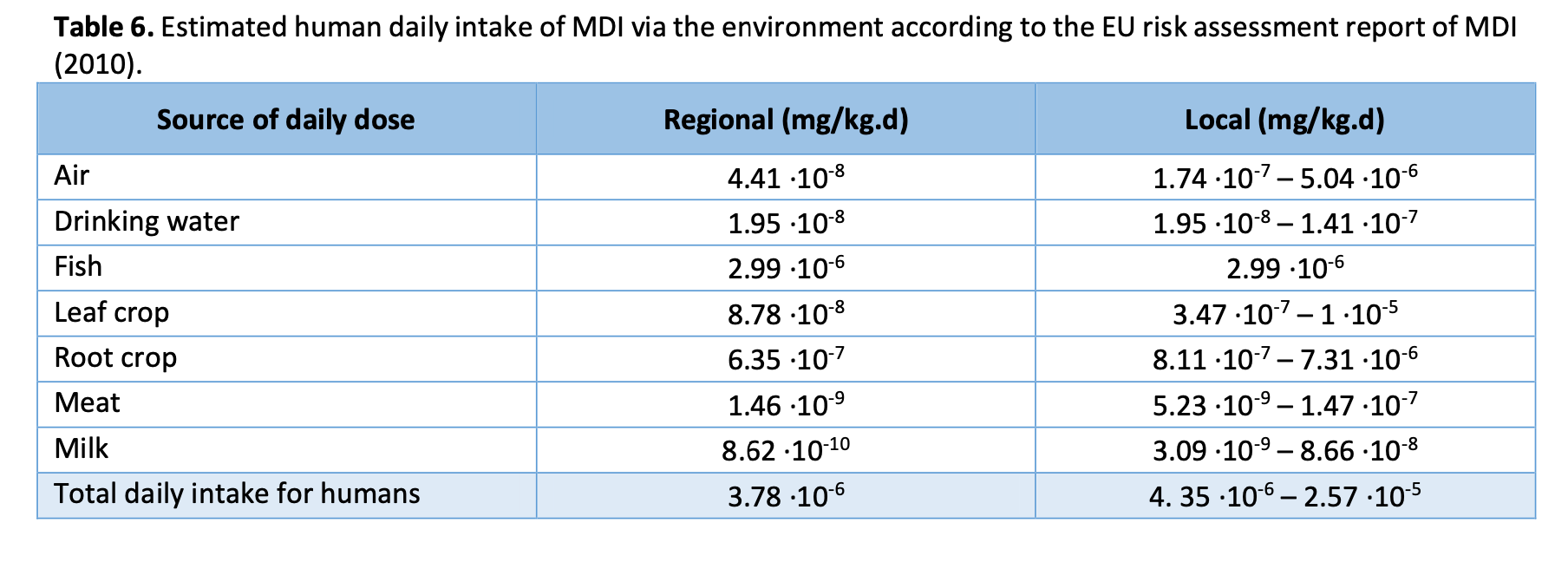
The EU risk assessment report of MDI also involves estimates of the daily human MDI dose through food, air and drinking water (see table 6) (European Commission 2010). The concentrations from environmental exposure are under normal circumstances so minor that they are not relevant even in long-term exposure. Furthermore, taking into account the high reactivity of isocyanates with water, human exposure to diisocyanates from water is very unlikely. Thereby accumulation through the aquatic food chain is very unlikely. (WHO 2000, European Commission 2010)
2.3 Dose-response relationship
Risk to human health will vary depending on the type and extent of exposure to isocyanates. However, there is insufficient human evidence about the exposure quantity - health effect relationship (WHO 2000). The health effects do not directly correlate to the severity of the exposure. In fact, based on findings in animal studies, lower doses can be more immunogenic and pathogenic than higher doses. In a mouse model, skin sensitisation with a lower dose of HDI resulted in substantially greater airway inflammation (Bello et al. 2004). In humans, sensitised individuals can develop allergy symptoms even at a very low level of exposure.
Because for the lack of knowledge about the human toxicokinetics, there is no harmonised international guidance for the exposure limits. In fact, the recommended occupational limits for isocyanates vary even within the EU. For example, the recommended exposure limit for MDI in 15 min is 0.035mg/m3 by the Finnish Institute of Occupation Health (TTL) and 0.07mg/m3 by the Health and Safety Executive (HSE) of the United Kingdom (see table 7). In comparison, even greater occupational exposure is allow in the United States: The occupational exposure limits for MDI is 0.2 mg/m3 in 10 min by the US National Institute for Occupational Safety and Health (NIOSH).
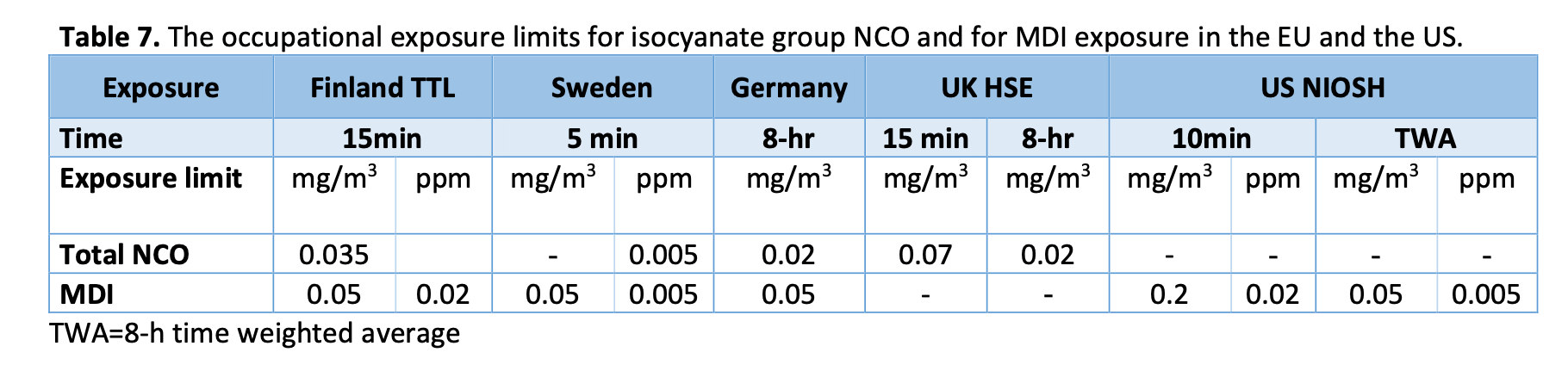
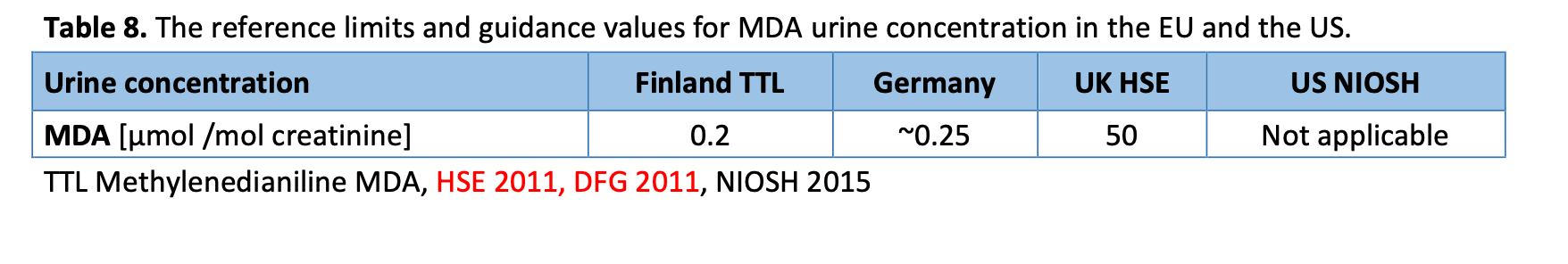
The concentration of MDA, a biological metabolite of MDI, in urine has been established to correlate with exposure to MDI (WHO 2000, European Commission 2010). Therefore occupational health can be regulated also by determining occupational reference limits for MDA concentration in urine. Similarly as occupational exposure limits for isocyanate exposure, these reference limits and guidance values vary between countries. The reference limit for exposure to MDA is set at 0.5 μmol per mole creatinine in urine by the Finnish TTL and at approximately 0.25 μmol per mole creatinine by German authorities. The guidance values by the UK HSE are a hundred times greater than the Finnish and two hundred times greater than the German values (see table 8).
2.4 Recognition and reporting of isocyanate exposure
The recognition of isocyanate exposure is lacking. The risk assessment in industrial sites handling isocyanates has been shown to be worse compared to industrial sites handling other chemicals. This was found in a study by the Finnish TTL, which investigated real-life occupational exposures of several chemicals in different industrial sites (n=70) involved in etc. rubber and plastic production. Sensitising chemicals caused a significant risk in 17 % (12/70) of industrial sites, and the causing agent was isocyanate in almost all of the cases. (TTL, Chemicals at work 2005)
Respiratory exposure to isocyanates appears to be better recognised than dermal exposure. Henriks- Eckerman et al. (2015) found that out of 24 workers handling MDI, 58 % did not use gloves at all, or used gloves that were not intended for chemical protection. In a study by the Finnish TTL, 11 out of 27 employees handling MDI used gloves protecting from chemical exposure, and 4 workers didn’t use gloves at all (Henriks- Eckerman et al. 2012).
The Finnish TTL also sent a questionnaire to healthcare professionals asking about the assessment of occupational diseases. Healthcare professionals stated that the most difficult occupational cause to assess was chemical risks at the work environment. More than 80 % of healthcare professionals felt that assessment of chemical exposure was between somewhat difficult or very difficult. According to the results, connecting the symptoms to the chemical cause was found to be difficult due to lack of information and large variety of used chemicals. Furthermore, healthcare professionals felt that the companies were not willing to co- operate. (TTL, Chemicals at work 2005)
With increased knowledge about the health hazards of isocyanates and implementation of exposure limits, there has been a downward trend of the incidence of isocyanate related occupational health issues. However, the Finnish Occupational Health Institute has estimated that the actual number of isocyanate related asthma can be 2-3 times the number of diagnoses.
There are several reasons why isocyanate exposure is often not recognized. The relationship between exposure and symptoms is undetected for example when allergy symptoms are delayed. This is related to the fact that downstream users are not aware of the potential health hazards of isocyanate exposure. The risk of isocyanate exposure is often well-known when working with high exposure products, e.g. spray painting. Therefore skin and respiratory protection are usually worn. However, the potential risks are not recognised in products containing minor proportions of isocyanates. Similarly short-term and occasional exposure can be thought to be less significant. Therefore some fast procedures, e.g. painting small items, can be done without wearing any protection. Sensitised subjects can also change assignments or employers without reporting occupational exposure to authorities. The management and recognition of exposure is also difficult because isocyanates cannot be detected by human senses. The detection limit for exposures is often 0.1 μg NCO/m3 in diagnostic methods. As a consequence, isocyanate exposure can be unidentified although it exists. (TTL, Chemicals at work 2005)
3. Isocyanates in casting work
In all orthopaedic synthetic casting tapes, MDI is used in polyurethane resins to help the resin turn from liquid to solid (Abbott, 2013). Estimates of the isocyanate concentration in synthetic plaster casts vary. In literature, modern plaster casts have been said to contain up to 25 % of MDI (Suojalehto et al. 2011) According to the material safety data sheet of 3MTM ScotchcastTM Soft Cast, an example of a casting product, the mass fraction of isocyanates in the fiberglass cast varies between 34-46 % (Material safety data sheet, 2009). In the product safety data sheet of another fiberglass cast product, Cellacast® Soft, the proportion of polyurethane prepolymer is announced to be 40 % (Product safety data sheet, 2009).
Today, the majority of orthopaedic casting work is made using fiberglass cast. As the fiberglass cast contain roughly a minimum of one third of MDI, the theoretical risk of isocyanate exposure in casting work is well- grounded. Even so, the occupational exposure to isocyanates in casting work is an unrecognised health risk.
3.1 Case-studies about isocyanate related asthma in casting workers
Several case studies have reported isocyanate related asthma in casting workers.
Suojalehto et al. (2011) published a case report of two nurses suffering from occupational asthma due to orthopaedic plaster casts containing MDI. One of the nurses had applied and removed synthetic plaster casts up to three times daily since 1990. She used latex or polyvinyl chloride gloves (PVC) while applying or removing casts. In 1994, a few hours after being involved in casting work, she developed a severe dyspnoea attack that was treated in an intensive care unit. She had suffered from mild rhinitis earlier. Asthma was diagnosed in 1999, but further examinations were done in 2006. She had three severe dyspnoea attacks and less severe symptoms regularly between 1999 and 2005. She had asthma medication when working. In 2006, a maximum of 25 % drop in forced expiratory volume in one second (FEV1) was observed in a specific inhalation test (given concentration 0.17 μg NCO/m3). Specific IgE tests and skin prick tests to diisocyanates were negative. The results confirmed occupational asthma and she was removed from casting work.
The other nurse had worked in an orthopedic operating room since 1998. Since 2003 he had applied and removed plaster casts up to two times daily. He used nitrile or latex gloves when applying but no gloves when removing casts. Six years afterwards he developed rhinitis, and work-related cough and dyspnea in the two following years. Asthma was diagnosed in 2007, and he was removed from casting work in 2008. Skin prick tests and specific IgE test to diisocyanates were negative. The specific bronchial test (concentration 8.6 μg NCO/m3) showed an early prolonged reaction and a maximum of 42 % drop in FEV1. The results confirmed occupational asthma. (Suojalehto et al. 2011)
In a case reported by Donnelly et al. (2003) a nurse developed cough, wheeze and dyspnoea on her return to MDI casting. She hadn’t worked with orthopaedic casts for a year, but had earlier worked with MDI- containing synthetic plaster casts on a daily basis for 4 years. The allergy symptoms began within five minutes of exposure. A bronchial provocation test was performed and confirmed an early asthmatic response.
Sommer et al. (2000) describe a case where a nurse developed rhinitis, itchy eyes and nightly wheezing during employment in an emergency room between 1990 and 1996. She had applied MDI-containing synthetic casts up to three times daily between 1990 and 1991. She had no history of atopy. She developed subsequent asthma attacks in 1992 and 1996. She developed a serious asthmatic attack after her husband used MDI- containing insulation foam. The nurse developed an asthma attack seven hours after a specific bronchial provocation test, with a 48 % fall in forced expiratory volume in one second (FEV1).
Tanaka et al. (1994) published a case where an orthopedist developed an early asthmatic response after a year of exposure to MDI-containing synthetic casts. He had a history of allergic rhinitis and conjunctivitis, and a family history of bronchial asthma and atopic dermatitis. He developed an asthmatic reaction while handling the casts, and a positive early response was confirmed on a bronchial challenge test. The scratch test for casting material was negative, but enzyme linked immunosorbent assay (ELISA) demonstrated the existence of specific IgG for MDI.
Based on published case reports, asthmatic reactions can be caused by occupational exposure to MDI- containing orthopaedic plaster casts. In all case reports, asthma symptoms began during several years of repeated exposure to MDI-containing plaster casts. Especially well-reported in the cases published by Suojalehto et al. (2011), it may be 10 years before asthmatic subjects are removed from casting work.
3.2 Estimates of isocyanate exposure during casting work
It is not completely reliable to estimate MDI concentration in orthopaedic casting tapes based on given product information. Casting tapes are stated to contain significant proportion of polyurethane polymer (30- 35 % mass faction). However, the amount of prepolymer is not necessary equal to amount of MDI. Prepolymer can contain unreacted NCO groups, and in fact it can contain less, more or equal proportion of NCO groups to monomeric MDI.
Only a few studies about evaluating the isocyanate exposure during casting work have been made. Suojalehto et al. (2011) estimated the exposure levels by measuring the diisocyanate concentration in the air during a casting simulation and during normal casting work in two hospitals. The samples were taken from the breathing zone (distance not specified) and above the casting zone (about 15 cm distance) when applying and removing the cast. The concentration in the breathing zone were found to be low, ranging 0.11-0.02 μg/m3 for NCO groups. In the nearcasting spot, the measured concentrations varied between 0.25 and 0.773 μg/m . For comparison, the occupational exposure limit by the Finnish Institute of Occupational Health is 3533 μg/m . The highest NCO concentration was measure in simulation removing plaster cast: 2.5μg/m. Compatible to that, sensitised nurses reported that skin contact appeared to be more frequent when removing the cast (Suojalehto et al. 2011).
In a study funded by 3M Company, Pearson et al. (2012) investigated a potential exposure to MDI in personnel applying orthopaedic casts. In casting stimulations, MDI concentration was measured from air, skin, working surfaces, and under gloves. In addition, concentrations of urinary metabolites of MDI (MDA) from 12-h urine collection were determined. No assessments were done during high-speed cutting during cast removal. According to the results, the MDI concentration in air sampling was below the quantitation limit 0.1μg. MDI was detected over 5μg on 1 of 60 skin samples, and on 4 of 45 surface samples taken. Unfortunately the precise values were given. The cast surface was estimated to be free of isocyanates in 20- 30 min after casting. Urine samples from six subjects’ exposure to MDI revealed MDA concentrations ranging 0.22-0.34 μmol MDA per mole creatinine. Interestingly, the average concentration of MDA in urine was higher when the casting tape was immersed in water before being applied (MDA detected in <80 % of subjects) vs. applied dry and sprayed with water after being applied (MDA detected in 50 % of subjects). (Pearson et al. 2012) The MDA concentrations in urine were above the reference limits of German Authorities and are approaching the reference value of TTL (see table 4). Especially in pregnant women these values can be significant risk for the unborn (TTL, methylenedianiline MDA).
According to the exposure estimates of the Finnish TTL, the respiratory exposure of MDI during 5-30min casting work is less than 0.1μg NCO/m3 (TTL, respiratory exposure). This estimate appears to be in line with the few studies done. However, no estimates about dermal exposure are available.
3.2.1 Evaluation of MDI exposure in casting work
Only a few studies have assessed the airborne concentrations of MDI while orthopaedic casting work. The detected airborne concentrations of MDI were found be very low. Based on the chemical properties, MDI has low volatility which supports airborne concentration being low (Henriks-Eckerman et al. 2015). However, activation of the plaster casts is exothermic reactions, which increases the local temperature and vaporisation of MDI. Factors influencing the extend of exothemicity are the temperature of the water bath and the degree of mechanical agitation imparted to the plaster (Donnelly et al. 2003).
Despite low detected airborne concentrations of MDI, it is difficult to assess the relevance of MDI concentration during casting work. For example in the study of Suojalehto et al. (2011), inhalation challenge was performed with the mean concentration of 0.17 μg NCO/m3, which is less than 5 % of the Finnish 15-min occupational exposure limit. The detection limit for exposures is often 0.1 μg NCO/m3. However, no threshold limit in sensitised subjects is known. Therefore, the risks of exposure can be unidentified although those exists.
As inhalation has long been considered the primary route of isocyanate exposure, the focus in exposure measurements is usually set on airborne concentrations. Also the Finnish TTL has given an estimate of the isocyanate exposure during casting work based on airborne exposure. However, the low airborne concentrations of MDI detected during casting work and several occupational asthmas observed in casting workers would suggest that dermal exposure could be the main source of MDI in casting work (Telephone discussion with Katriina Ylinen). In fact, Henriks-Eckerman et al. (2015) concluded that dermal exposure might be an important route in systemic intake of MDI among workers in construction and boat building industry. The risk of dermal exposure increases in casting work, where the risk of diisocyanates is unrecognised and unappropriated protecting materials are worn.
3.3 Working practices in casting work
Despite good-quality publications about isocyanate related asthma in casting workers and studies about protecting work wear, the results have not been implemented to good working practices. In safety data sheets of casting tapes, “use of protecting gloves” is recommended. However, not all gloves available in hospital environment protect from diisocyanate exposure. In fact, there has been several studies investigating permeation of a wide range of working materials and gloves to diisocyanates. In casting work, it should be noted that latex gloves have been demonstrated to have significant permeation by isocyanate monomers. In addition, also vinyl gloves have demonstrated high leakage rates. Nitrile gloves have not been found to permeate MDI. (Pearson et al. 2012) In a study of the Finnish TTL, the most common working cloth materials were found to easily permeate diisocyanates (Henriks-Eckerman et al. 2012).
The Finnish TTL has not set any specific occupational health guidelines for casting workers: no preventive follow-up visits or spirometry is recommended (Leino et al. 2014). According to the Swedish working environment regulations, individuals influenced to isocyanates should be properly trained for its risk. The Swedish Work Environment Authority has stated that such individuals should visit a doctor for regular examination of respiratory function. The Swedish guidance concerning isocyanates concludes that less harmful products should be used instead of products containing isocyanates whenever available. (Swedish Work Environment Authority; AFS 2005:18, AFS 2005:6)
While writing this report it became clear that receiving material safety data sheets of casting products’ from retailers is arduous. Furthermore, it also turned out that a responsible person for sales of casting products, in a multinational company, did not understand why a product like casting tape would even need a product safety data sheet. She also said that this type of information is confidential property of the manufacturer. (Telephone and email discussion with Mediq Oy) This raises a question, are the end-users of casting products really properly informed and trained about potential health risks. This suspicion was further confirmed in telephone discussion with Katriina Ylinen, special expertise in chemical occupational hygiene, from the Finnish TTL. She had been involved in measuring airborne isocyanate concentrations while casting (Suojalehto et al. 2011). According to her experience, casting workers are not aware of the potential risk of casting products neither the permeation tests of glove and clothing materials. Therefore they usually use gloves easiest available, and not specifically nitrile gloves.
The use of orthopaedic plaster casts is entrenched while the potential health risks in casting work are not recognized. In general, casting workers appear not to be aware of plaster casts containing isocyanates. There are no guidelines, good working practices or product safety data provided to casting workers. Despite studies about appropriate gloves and working clothes, the results concerning materials not permeating isocyanates have not been implemented on good working practices. In fact, casting workers are thought to use gloves that are easily available, despite only nitrile gloves have been shown to protect from diisocyanate exposure. Due to complete lack of awareness of health risks in casting work, the proper protection while casting is unlikely.
As a conclusion, MDI exposure in casting work could be easily limited by increasing the awareness of diisocyanates possible health effects and advising casting workers to wear appropriate protection.
References
Aalto-Korte K, Suuronen K, Kuuliala O, Henriks-Eckerman ML, Jolanki R. Occupational contact allergy to monomeric isocyanates. Contact Dermatitis. 2012 Aug;67(2):78-88.
Abbott Richard. Casting –Health and Safety. Isocyanate. 2013. Available from: http://www.fannin.eu/wp- content/uploads/2013/03/1Health-Safety-ISOCYANATE-MDI.pdf
Bello D, Herrick CA, Smith TJ, Woskie SR, Streicher RP, Cullen MR, Liu Y, Redlich CA. Skin exposure to isocyanates: reasons for concern. Environ Health Perspect. 2007 Mar;115(3):328-35.
Bello D, Woskie SR, Streicher RP, Liu Y, Stowe MH, Eisen EA, Ellenbecker MJ, Sparer J, Youngs F, Cullen MR, Redlich CA. Polyisocyanates in occupational environments: a critical review of exposure limits and metrics. Am J Ind Med. 2004 Nov;46(5):480-91.
Broughton E. The Bhopal disaster and its aftermath: a review. Environ Health. 2005; 4: 6.
CDC, Centers for Disease Control and Prevention. Work place safety & health topics. Isocyanates. Available from: http://www.cdc.gov/niosh/topics/isocyanates/. Last updated: Nov. 2014. Cited Feb 2015.
Creely KS, Hughson GW, Cocker J, Jones K. Assessing isocyanate exposures in polyurethane industry sectors using biological and air monitoring methods. Ann Occup Hyg. 2006 Aug;50(6):609-21. Epub 2006 May 26.
Dhara R. Health effects of the Bhopal gas leak: a review. [Abstract] Epidemiol Prev. 1992 Sep;14(52):22-31. European Commission. Joint Research Centre. Summary of Risk Assessment Report. European Communities. 2010
Finnish Institute of Occupational Health. [Chemicals at work – Report of the Finnish Institute of Occupational Health for the National Programme on Chemical Safety]. [Report in Finnish]. Työterveyslaitos 2005.
HESIS, Hazard Evaluation system & Information service. Isocyanates: Working Safely. California Department of Public Health. 2014
Henriks-Eckerman ML, Mäkelä E, Puttonen K, Laitinen J, Vuokko A, Suuronen K, Mannelin T, Tuomi T, Sauni R. Ihonsuojauksen ja turvallisten työtapojen merkitys MDI-uretaanityössä. Loppuraportti. Työterveyslaitos 2012.
Henriks-Eckerman ML, Mäkelä EA, Laitinen J, Ylinen K, Suuronen K, Vuokko A, Sauni R. Role of dermal exposure in systemic intake of methylenediphenyl diisocyanate (MDI) among construction and boat building workers. Toxicol Lett. 2015 Feb 3;232(3):595-600.
Hoffmann HD, Leibold E, Ehnes C, Fabian E, Landsiedel R, Gamer A, Poole A. Dermal uptake and excretion of 14C- toluene diisocyante (TDI) and 14C-methylene diphenyl diisocyanate (MDI) in male rats. Clinical signs and histopathology following dermal exposure of male rats to TDI. Toxicol Lett. 2010 Dec 15;199(3):364-71.
Liljelind I, Norberg C, Egelrud L, Westberg H, Eriksson K, Nylander-French LA. Dermal and inhalation exposure to methylene bisphenyl isocyanate (MDI) in iron foundry workers. Ann Occup Hyg. 2010 Jan;54(1):31-40.
Leino T, Rautio M, Kanervisto M, Tilli J, Kaleva S. Terveystarkastuskäytännöt suomalaisessa työterveyshuollossa. Työterveyslaitos TTL. 2014
Material Safety Datasheet. 3MTM ScotchcastTM Soft Cast. 3M Company. 2009.
Mhike M, Chipinda I, Hettick JM, Simoyi RH, Lemons A, Green BJ, Siegel PD. Characterization of methylene diphenyl diisocyanate-haptenated human serum albumin and hemoglobin. Anal Biochem. 2013 Sep 15;440(2):197-204.
NCDOL, N.C. Department Labor. A guide to occupational exposure to isocyanates. N.C. Department of Labor. Occupational Safety and Health Program. 2013. Available from: http://www.nclabor.com/osha/etta/indguide/ig46.pdf
NIOSH. NIOSH Pocket Guide to Chemical Hazards. Department of Health & Human Services, Centers for Disease Control & Prevention. National Institute for Occupational Safety & Health. DHHS (NIOSH) Publication No. 2010-168 (2010). Available from: http://www.cdc.gov/niosh/npg
Pearson RL, Logan PW, Kore AM, Strom CM, Brosseau LM, Kingston RL. Isocyanate exposure assessment combining industrial hygiene methods with biomonitoring for end users of orthopedic casting products. Ann Occup Hyg. 2013 Jul;57(6):758-65.
Product safety data sheet. Cellacast® Soft. Lohmann and Rauscher. 2009.
Sommer BG, Sherson DL, Kjøller H, Hansen I, Clausen G, Jepsen JR. [Asthma caused by methylene-diphenyl- diisocyanate cast in a nurse.] [Article in Danish] Ugeskr Laeger. 2000 Jan 24;162(4):505-6.
Suojalehto H, Linström I, Henriks-Eckerman ML, Jungewelter S, Suuronen K. Occupational asthma related to low levels of airborne methylene diphenyl diisocyanate (MDI) in orthopedic casting work. Am J Ind Med. 2011 Dec;54(12):906- 10.
Sullivan JB, Krieger GR. Clinical Environmental Health and Toxic Exposures. Lippincott Williams & Wilkins, 2001 Swedish Work Environment Authority. Arbetsmiljöverkets föreskrifter om härdplaster, AFS 2005:18.
Swedish Work Environment Authority. Arbetsmiljöverkets föreskrifter och allmänna råd om medicinska kontroller i arbetslivet. AFS 2005:6
Tanaka Y, Satoh F, Komatsu T, Muto H, Akiyama N, Arai Y, Miyamoto Y, Sano Y. [A case of suspected occupational asthma in an orthopedist, due to cast materials containing MDI]. [Article in Japanese] Nihon Kyobu Shikkan Gakkai Zasshi. 1994 Jun;32(6):606-9.
Telephone and email discussions with Marja Nykyri-Weckman, Manager of Customer Services. Mediq Suomi Oy. March 13th, 2015.
Telephone discussion with Katriina Ylinen, specialist in chemical working hygiene, The Finnish Institute of Occupational Health. March 18th, 2015.
Telephone and email discussion with Irmeli Lindström, The Finnish Institute of Occupational Health. March 9th and 18th, 2015.
TTL, the Finnish Institute of Occupational Health. [Biomonitoring of chemical exposures. Guidance for sampling] [Available in Finnish]. 17.painos. Työterveyslaitos. 2013.
TTL, The Finnish Institute of Occupational Health. Chemicals at work – Report of the Finnish Institute of Occupational Health for the National Programme on Chemical Safety. Työterveyslaitos. 2005.
TTL, the Finnish Institute of Occupational Health. [Isocyanates, polyurethanes and their use in different industrial sectors.] [Available in Finnish] Last updated: Jan 2013. Cited Feb 2015. Available at: http://www.ttl.fi/fi/kemikaaliturvallisuus/ainekohtaista_kemikaalitietoa/isosyanaatit/isosyanaatit_polyuretaanit_%20 ja_niiden_k%C3%A4ytt%C3%B6_eri_aloilla/sivut/default.aspx
TTL, the Finnish Institute of Occupational Health. [Health effects of isocyanates] [Website available in Finnish] Last updated: Jul 2013. Cited Feb 2015. Available from: http://www.ttl.fi/fi/kemikaaliturvallisuus/ainekohtaista_kemikaalitietoa/isosyanaatit/isosyanaattien_aiheuttamat_ter veyshaitat/sivut/default.aspx
TTL, the Finnish Institute of Occupational Health. [Methylenedianiline MDA.] [Available in Finnish]. Available from: http://www.ttl.fi/fi/palvelut/turvallisempi-tyoymparisto/biomonitorointi/Documents/Metyleenidianiliini_MDA.pdf
TTL, the Finnish Institute of Occupational Health. [Respiratory exposure to isocyanates in urethane work. Exposure levels and prevention]. [Available in Finnish]. Available from: http://www.ttl.fi/fi/kemikaaliturvallisuus/ainekohtaista_kemikaalitietoa/isosyanaatit/isosyanaateille_altistuminen_ris kienhallinta/Documents/nco_hengitystiealtistuminen_torjunta.pdf
Verschoor L, Verschoor AH. Nonoccupational and occupational exposure to isocyanates. Curr Opin Pulm Med. 2014 Mar;20(2):199-204.
WHO. World Health Organisation. Concise International Chemical Assessment Document 27. Diphenylmethane diisocyanate (MDI). 2000
Anal Biochem. 2010 May 15; 400(2): 251–258.
Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010 May 15;400(2):251-8.
Wisnewski AV, Liu J, Colangelo CM. Glutathione Reaction Products with a Chemical Allergen, Methylene-diphenyl Diisocyanate, Stimulate Alternative Macrophage Activation and Eosinophilic Airway Inflammation. Chem Res Toxicol. 2015 Feb 18.
